Abstract
Background: Patients with acute myeloid leukemia (AML) may experience substantial disease- and treatment-related symptoms. Patient-reported outcome (PRO) assessments are validated tools to evaluate patient symptoms and function, and are associated with improved health-related quality of life (HRQoL), reduced unplanned acute care, and longer overall survival among patients with advanced solid tumors (Basch E et al. JAMA, 2017.318(2):197-198). However, PROs are used infrequently in the AML population outside of clinical trials.
Objectives: We assessed the characteristics of baseline PRO measurements in patients with newly diagnosed AML and evaluated their association with short-term acute care utilization and receipt of allogeneic hematopoietic stem cell transplantation (alloHSCT).
Methods: We retrospectively identified all patients with non-M3 AML diagnosed between 6/2018 and 3/2020 who were actively receiving therapy at our institution and completed at least one outpatient PRO survey within a period of 1 month preceding to 3 months following AML diagnosis. This assessment period was selected to allow us to assess outpatient PROs, since many patients are admitted at initial presentation and remain inpatient for an extended period at the time of diagnosis. The survey completed at the time closest to the time of diagnosis was defined as baseline PRO. PROs were measured using a 14-question assessment administered before each outpatient encounter using the National Cancer Institute's PRO Version of The Common Terminology Criteria for Adverse Events. The survey addresses 9 symptom scales rated by patients on a scale of 1-5 ranging from no symptom to very severe symptom with higher scores indicating worse symptoms, and 1 QoL scale with higher scores indicating improved QoL. Time to acute care visit (TAC) was defined as the time from baseline PRO measurement to the first unplanned acute care visit (emergency room visit or unplanned hospitalization) and assessed using Kaplan-Meier survival function and log-rank test. Fisher's exact test was used to assess the associations between symptom burden and age, therapy intensity subgroups, and alloHSCT status.
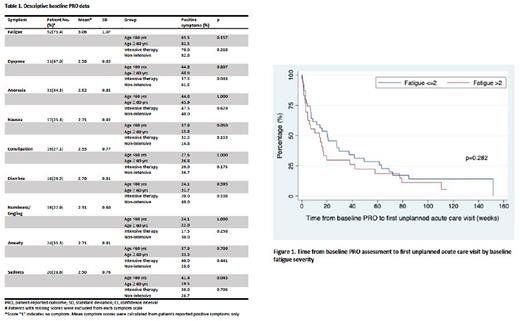
Results: Seventy patients with a median age of 63 years (range 22-88) at AML diagnosis were included in the analysis. The median follow-up was 19.2 months. Forty patients (57.1%) underwent intensive induction therapy, i.e., "7+3" and its variations. Thirty patients (42.9%) received non-intensive therapy, primarily venetoclax/azacitidine. The most common (n=52, 75.4%) and most severe symptom reported in our cohort was fatigue, with a mean score of 3.06 (standard deviation (SD) 1.07). Symptom burden did not differ between younger (< 60yrs (n=29, 41.4%)) and older (≥ 60yrs) patients, except for sadness which was reported more frequently in patients < 60yrs (41.4% vs. 19.5%, p=0.043). Patients undergoing non-intensive therapy reported more frequent dyspnea at baseline (61.5% vs. 37.5%, p=0.048) (table 1). Mean QoL score (2.71, SD 1.21) did not differ by age or induction intensity. The median TAC was 16.1 weeks with 60 (85.7%) patients having at least one acute care visit. Patients with moderate to severe baseline fatigue (fatigue score > 2 (n=30, 43.5%) ) had a median TAC of 13.9 weeks and tended to a higher risk of sooner acute care visit initially compared to those with none to mild fatigue (fatigue score ≤ 2) (TAC 20.4 weeks, p=0.282) (figure 1). In patients with intermediate- and adverse-risk disease (n=54), more patients with baseline fatigue score ≤ 2 (n=28, 51.9%) were able to undergo alloHSCT (53.6% vs. 28.0%, p=0.053). Additionally, lower fatigue scores on follow-up PRO survey post-induction were associated with a higher rate of alloHSCT (61.5% for patients with fatigue scores ≤ 2 vs. 24.0% with fatigue scores >2, p=0.007) despite no difference in remission status between the two groups post-first induction cycle (53.6% vs. 31.8%, p=0.182).
Conclusions: In patients with newly diagnosed AML, fatigue was the most prominent symptom reported at baseline. Patients with worse baseline fatigue may be at risk for sooner unplanned acute care visits within the first year of diagnosis. Patients with less fatigue post-induction had a higher chance of undergoing alloHSCT. Further investigation is needed to better understand and utilize the real-world PRO data to help provide patient-centered, goal-concordant care to AML patients.
Disclosures
Perl:Astellas, Abbvie, Daiichi Sankyo, FujiFilm, Syndax: Research Funding; Astellas, Daiichi Sankyo, Abbvie, Genentech, BerGenBio, Immunogen, BMS/Celgene, Actinium: Membership on an entity's Board of Directors or advisory committees; Astellas, Daiichi Sankyo, AbbVie, Forma, Sumitomo Dainippon, BeatAML LLC, Loxo, LLS/Beat AML, Forma, New Link Genetics, Bayer, Biomed Valley Discoveries: Consultancy. McCurdy:Bristol Myers Squibb: Consultancy. Frey:Sana Biotechnology, Kite Pharma, and Syndax Pharmaceuticals: Consultancy; Novartis: Research Funding. Hexner:Tmunity Therapeutics: Research Funding; PharmaEssentia: Consultancy; Blueprint Medicines Corporation: Consultancy, Research Funding; Samus Therapeutics, Novartis Oncology: Research Funding; American Board of Internal Medicine: Other: Member of the hematology exam committee. Babushok:PHAR, LLC: Consultancy; Carisma Therapeutics: Current equity holder in private company. Porter:Incyte: Consultancy; Novartis: Consultancy, Patents & Royalties: anti-CD19 CART, Research Funding; Kite/Gilead: Consultancy; Gerson Lerhman Group: Consultancy; Janssen: Consultancy; Jazz: Consultancy; Adecept Bio: Consultancy; DeCART: Consultancy; BMS: Consultancy; Bluebird Bio: Consultancy; Kadmon: Consultancy; Angiocrine: Consultancy; Mirror Biologics: Consultancy; Genentech: Current equity holder in publicly-traded company; Roche: Current equity holder in publicly-traded company; Tmunity Therapeutics: Patents & Royalties: anti-CD19 CART; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Wiley: Honoraria; Elsevier: Honoraria. Pratz:AbbVie, Agios, Daiichi Sankyo, Millennium: Research Funding; AbbVie, Astellas, Boston BioMedical, BMS, Celgene, Novartis, Jazz Pharmaceuticals, and Servier.: Membership on an entity's Board of Directors or advisory committees. Lai:Astellas, Jazz: Speakers Bureau; AbbVie, Agios/Servier, Daiichi-Sankyo, Jazz, Macrogenics, PDS, Pfizer, Genentech, Taiho, Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal